Orthopedic surgeries play a critical role in addressing various musculoskeletal issues, enhancing patients’ quality of life, and enabling a return to normal activities. However, not all medical devices, drugs, biologics, and therapies used in these procedures are without controversy. In this article, we will delve into the history, applications, and concerns surrounding ten controversial orthopedic devices, drugs, and biologics, as well as the debated Platelet-Rich Plasma (PRP) therapy, and highlight the Mako Total Knee system.
Metal-on-metal hip implants

Metal-on-metal hip implants were first introduced in the 1960s, offering improved durability and longevity compared to other materials. However, concerns began to emerge in the late 2000s as patients experienced complications like metallosis, caused by metal debris entering the bloodstream and surrounding tissue. High failure rates and a higher likelihood of revision surgeries led to increased scrutiny of these implants. Despite the controversy, some surgeons continue to use metal-on-metal hip implants, citing the need for individualized patient care.
Bone graft substitutes
Bone graft substitutes were developed to address the limitations of autografts and allografts, such as donor site morbidity and limited availability. Synthetic bone graft substitutes have faced scrutiny due to potential complications like increased infection risk and poor integration with surrounding bone. Despite these concerns, many surgeons continue to use bone graft substitutes, as they offer numerous benefits, including reduced operative time and lower morbidity.
Pain pumps
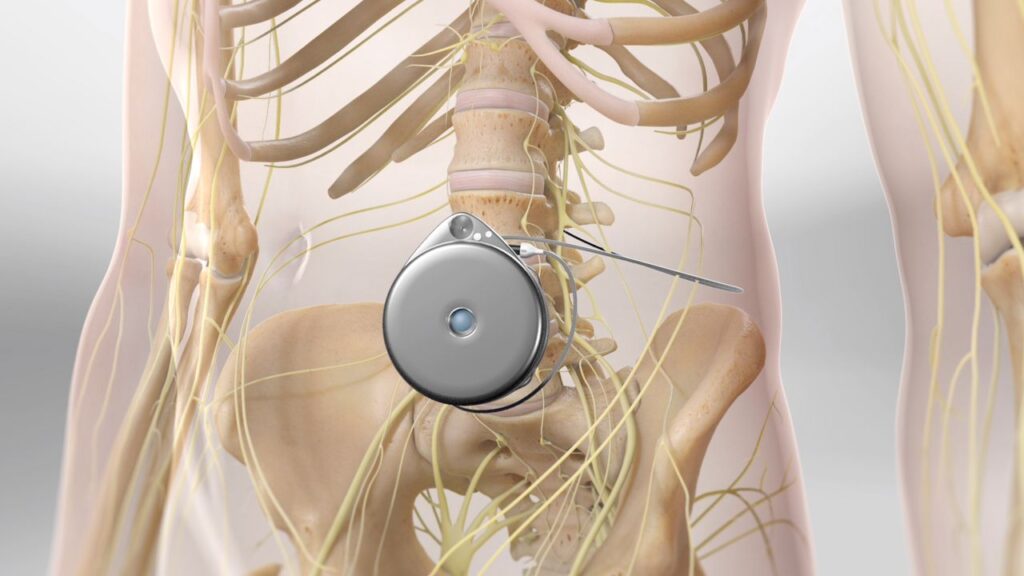
Pain pumps were developed as a method of delivering pain medication directly to the surgical site, offering patients a more controlled and targeted pain management option. However, concerns arose when some patients experienced chondrolysis (cartilage damage) after arthroscopic shoulder surgery. The FDA has since issued a warning about the potential risks associated with pain pumps, leading many surgeons to adopt alternative pain management strategies.
Infuse Bone Graft (BMP-2)
The Infuse Bone Graft, a recombinant human Bone Morphogenetic Protein-2 (rhBMP-2), was approved by the FDA in 2002 for use in anterior lumbar interbody fusion (ALIF) procedures. However, concerns emerged about potential complications, including ectopic bone growth, nerve damage, and increased cancer risk. Despite these concerns, BMP-2 remains in use for specific applications and has been shown to promote bone fusion effectively.
Transvaginal mesh
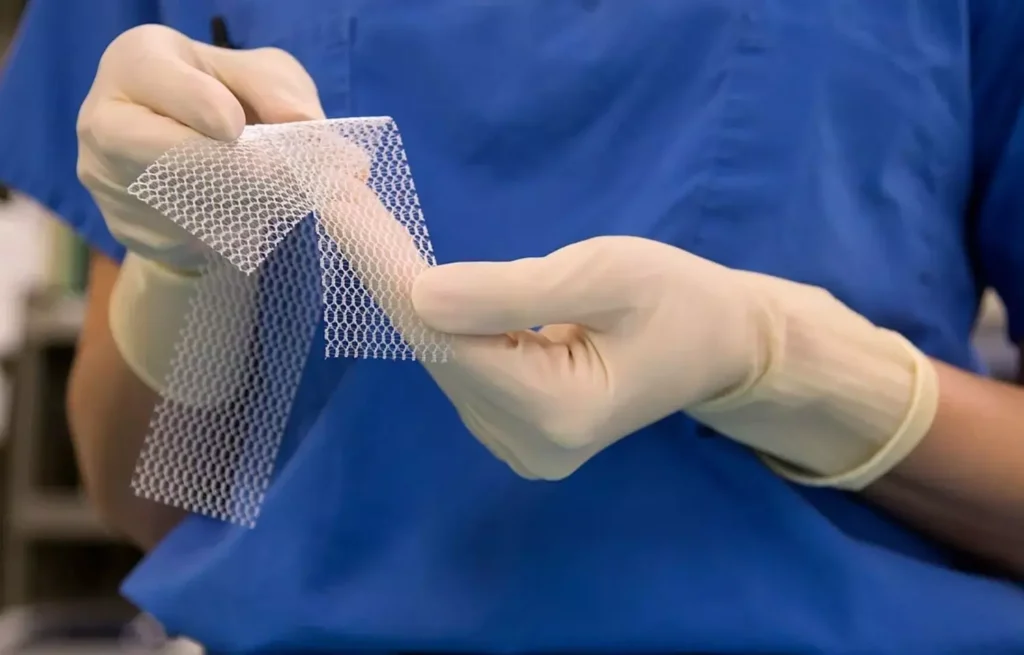
Transvaginal mesh, a synthetic material used to treat pelvic organ prolapse and stress urinary incontinence, gained popularity in the early 2000s. While not an orthopedic device, it has faced numerous lawsuits due to complications like mesh erosion, chronic pain, and infection. The FDA reclassified transvaginal mesh as a high-risk device in 2016, and multiple manufacturers have since stopped selling the product.
DePuy ASR Hip Resurfacing System
The DePuy ASR Hip Resurfacing System, a metal-on-metal hip implant, was introduced in the early 2000s. However, high failure rates and increased revision surgeries led to a voluntary recall by the manufacturer in 2010. Despite the recall, some surgeons argue that the ASR system provided satisfactory results for select patients, emphasizing the importance of patient selection and surgical technique.
Zimmer NexGen Knee Implant
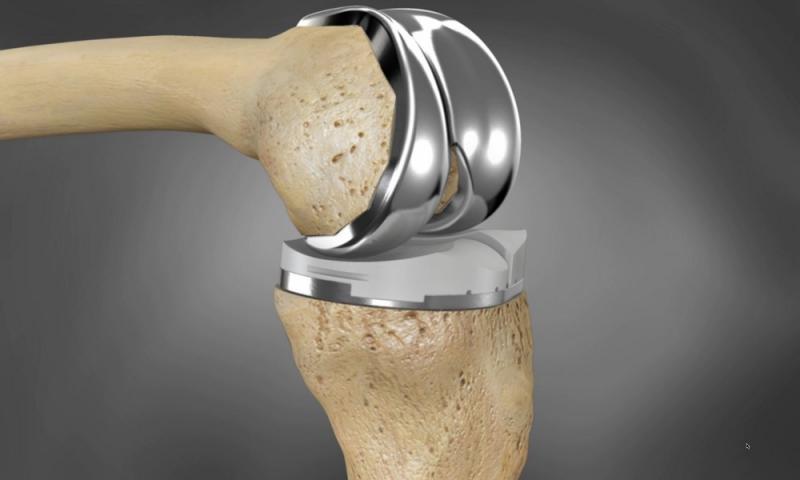
The Zimmer NexGen Knee Implant system, introduced in the mid-1990s, has faced criticism for alleged high failure rates and the need for revision surgery in some cases. Critics argue that the design of the implant may contribute to these issues, while proponents maintain that patient selection and surgical technique are critical factors. In recent years, Zimmer Biomet has developed robotic-assisted technologies like the Mako Total Knee system, which aims to improve patient outcomes by offering personalized surgical planning and precise implant placement.
Stryker Rejuvenate and ABG II Modular-Neck Hip Stems
Stryker’s Rejuvenate and ABG II Modular-Neck Hip Stems, introduced in 2009 and 2010, respectively, were designed to provide surgeons with greater customization options for hip replacements. However, fretting and corrosion at the modular neck junction led to adverse tissue reactions and the need for revision surgery in some cases. Stryker voluntarily recalled both systems in 2012, and since then, they have focused on improving their product line with innovations like the Mako Robotic-Arm Assisted Surgery System.
Wright Medical Conserve Hip Implant
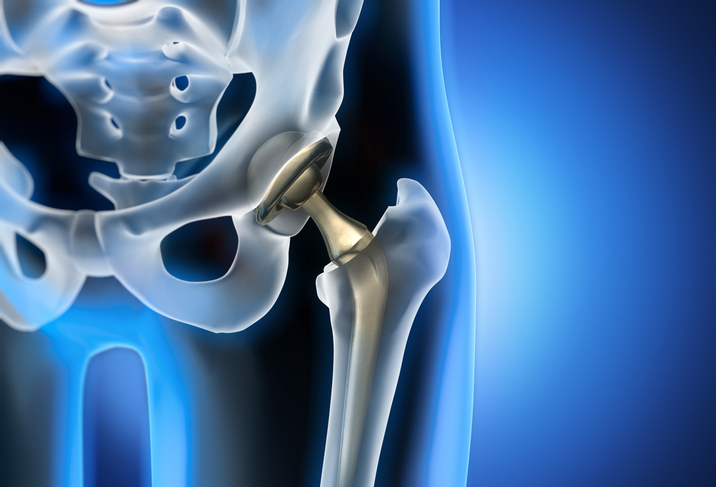
The Wright Medical Conserve Hip Implant, another metal-on-metal hip implant system, faced scrutiny due to alleged high failure rates and complications related to metal debris. While the FDA never issued a recall for the Conserve Hip Implant, the manufacturer eventually discontinued the product due to mounting legal issues and negative publicity. Wright Medical has since shifted its focus to other orthopedic solutions and technologies.
Biomet M2a Magnum Hip Implant
The Biomet M2a Magnum Hip Implant, a metal-on-metal hip implant system, faced concerns over high failure rates and complications related to metal debris. Despite the controversies, the M2a Magnum system remains on the market, with some surgeons advocating for its use in specific cases. However, Biomet (now Zimmer Biomet) has continued to invest in research and development to offer a range of hip implant options for surgeons and patients.
Platelet-Rich Plasma (PRP) Therapy
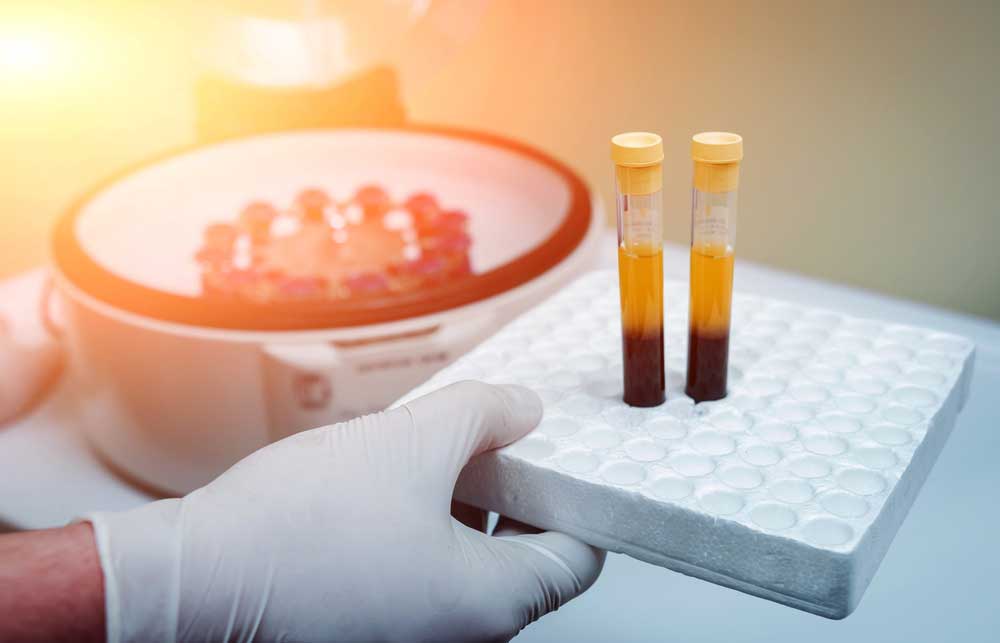
Platelet-Rich Plasma (PRP) therapy is a regenerative medicine technique that has gained popularity in the orthopedic field in recent years. PRP therapy involves drawing a small sample of the patient’s blood, processing it in a centrifuge to separate and concentrate the platelets, and then injecting the platelet-rich plasma into the injured area to promote healing and tissue regeneration.
PRP therapy was first introduced in the 1970s for dental and oral surgery procedures but has since expanded to various medical specialties, including orthopedics, sports medicine, and pain management. In orthopedics, PRP has been used to treat a wide range of conditions, such as tendonitis, osteoarthritis, ligament injuries, and muscle strains. It has also been employed as an adjunct therapy in surgical procedures like rotator cuff repair, ACL reconstruction, and joint replacement surgery to enhance healing and recovery.
While PRP therapy has shown promising results in some cases, its efficacy remains a topic of debate among medical professionals. Several factors contribute to the controversy surrounding PRP therapy:
• Inconsistency in preparation and administration: PRP preparation protocols and injection techniques can vary significantly, leading to inconsistency in the concentration of platelets, growth factors, and other bioactive components in the final product. This variability makes it difficult to compare study results and draw definitive conclusions about PRP’s effectiveness.
• Lack of standardized guidelines: The absence of standardized guidelines for PRP preparation, administration, and dosing contributes to the uncertainty surrounding its efficacy. As a result, healthcare providers may use different PRP formulations and treatment protocols, making it challenging to determine the most effective approach.
• Limited high-quality research: While numerous studies have been conducted on PRP therapy, many suffer from small sample sizes, lack of control groups, or other methodological limitations. This makes it difficult to establish strong evidence for PRP’s effectiveness and identify best practices for its use. In addition, some studies have found no significant benefits of PRP therapy compared to placebo or other treatments, further fueling the debate.
• Cost and insurance coverage: PRP therapy can be expensive, and insurance coverage for the treatment varies significantly among providers and plans. The lack of universally accepted clinical guidelines and solid evidence supporting PRP’s efficacy has led some insurers to consider the treatment experimental, limiting patients’ access to the therapy.
The orthopedic field is continually evolving, with innovations in devices, drugs, biologics, and therapies designed to improve patient outcomes. However, some of these advances have been met with controversy, as concerns arise regarding their safety, efficacy, and long-term consequences. It is crucial for clinicians, researchers, and regulatory bodies to continually evaluate and scrutinize these treatments, weighing the potential benefits against the risks.
In the case of the Mako Total Knee system, this robotic-assisted technology has shown promise in improving patient outcomes by offering personalized surgical planning and precise implant placement.
However, long-term studies and data are necessary to validate its effectiveness fully.
Similarly, PRP therapy has generated significant interest and debate within the orthopedic community. While some studies have demonstrated positive results, others have found no significant benefits. The lack of standardized guidelines and high-quality research has made it challenging to determine PRP’s true effectiveness.
Ultimately, continued research, development, and scrutiny of these controversial orthopedic devices, drugs, biologics, and therapies are essential to ensure that patients receive the best possible care and outcomes. As our understanding of these treatments grows, so too will our ability to provide effective, personalized care for those suffering from musculoskeletal conditions.






